Introduction
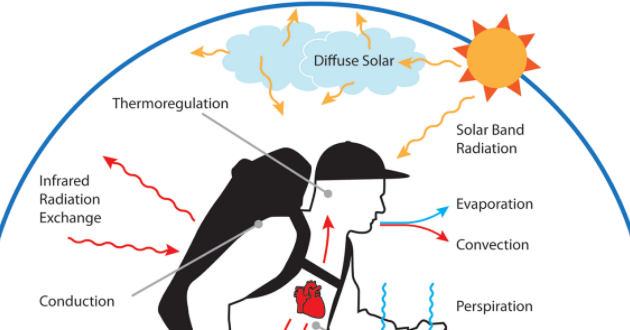
One of the most important physiological constraints that have impacted the evolution of the human lineage is thermoregulation. Thermoregulation is any physiological or behavioural mechanism that acts to retain or generate body heat in environments colder than body temperature in order to prevent hypothermia, or that acts to remove body heat in environments warmer than body temperature in order to prevent hyperthermia.
There are two main categories used to describe species with different body temperature systems, poikilotherms and homeotherms. Poikilotherms are organisms whose body temperatures are relatively variable and change with the changing environmental temperature. Homeotherms are organisms that maintain their body temperature within a very narrow range through a variety of thermoregulatory structural and physiological devices in widely varying environmental temperatures.
Homo sapiens is a homeothermic species that must maintain a relatively stable body temperature no matter what environmental conditions individuals are exposed to. Humans differ in many important aspects from other primate species in the structure and function of their thermoregulatory devices, and these changes are most probably related to the evolutionary pressures our ancestors faced at some time in the past.
This chapter will describe the structure and function of the modern human thermoregulatory system, and place it in a comparative context with other closely related primate species. The possible selective pressures that likely faced our ancestors will be discussed in an explanatory framework under which certain features of the modern human condition could have evolved. The chapter will end with a discussion of various hypotheses that have been put forth to answer questions regarding the evolution of the human thermoregulatory system.
Heat Loss and Retention
The function of the thermoregulatory system of a homeothermic organism is to maintain a constant core body temperature under different environmental conditions. This involves mechanisms and adaptations that prevent excessive heat loss or that produce heat in environments colder than the core body temperature, and mechanisms and adaptations that prevent excessive heat gain or that remove heat in environments warmer than the core body temperature. These mechanisms and adaptations involved in thermoregulation act to balance thermal inputs and thermal losses.
Thermal input comes from a variety of sources that include the air or water temperature of the environment when the temperature is greater than body temperature, radiant solar energy or radiant energy reflected from sources such as the ground or rocks, and the internal metabolic heat load from physiological functions. Thermal loss comes from a variety of sources as well, including convection to the environment when the temperature is lower than body temperature and evaporation across mucous membranes or through sweating.
The thermal input from convection between the environment and the skin of an individual is important to heat stress when the thermoregulatory system is working to remove heat, but it is not involved in the system to retain heat, as input will only occur if the ambient environmental temperature is greater than the temperature of the core body temperature. So while not an important heat source when trying to maintain body temperature in the face of excessive heat loss, it is an important stressor upon the thermoregulatory system when faced with excessive heat gain.
The second important environmental heat input is from direct and indirect solar radiation. Solar radiation can be broken up into long-wave and short-wave radiation. Long-wave radiation is absorbed equally regardless of skin or hair colour, while short-wave radiation absorption depends on the colour of the skin or hair of the organism (the darker the colour, the greater the absorption). The heat input from this source can account for a significant proportion of thermal stress is some environments where the organism is being bombarded by direct solar radiation (Wheeler 1991a).
A major source of thermal input is metabolic heat load from various physiological functions. The metabolic heat load can actually be up to ten times that of the environmental heat load when under sustained aerobic metabolic stress in an intermediate to large-bodied homeotherm (Taylor 1977). Sources of metabolic heat include metabolic thermogenesis, contractile thermogenesis, and the lipolysis with the oxidation of fatty acid (Chatterjee 1979). Metabolic thermogenesis occurs as glucose or lipids are metabolized by the citric acid cycle or in other metabolic pathways as waste heat is produced as chemical reactions occur. This metabolic source is generally greatest due to the concentration of the largest mass of metabolically active tissue.
The brain also has a very high metabolic requirement and therefore produces a large amount of waste heat through action potential, synaptic, and membrane transport activity. Contractile thermogenesis is the production of frictional heat that occurs with the stretching of the elastic muscles or tendons. When a muscle is contracted, only about 22% of the energy produced by metabolic activity goes towards the work of the muscle, the rest appears as heat energy (Sanyal & Maji 2001). Lipolysis is the breakdown of fatty tissue with resulting production of metabolic heat. This is extremely important in colder environments and is an important process when considering the evolution of human heat stress adaptations and the resulting weakness to cold stress.
Just as convective heat gain from the environment is less of a thermoregulatory input than it is a stress on the system, convective heat loss to the environment is a stress on the thermoregulatory system when the organism needs to retain heat in colder environments. The heat transfer across the skin to the environment can be great and is an important factor in human thermoregulation. Besides convection with the environment, evaporation is the only heat loss mechanism available physiologically to humans. Evaporation takes place in the lungs and nasal chambers across mucous membranes in many homeotherms and some behavioural adaptations like saliva spreading can also increase the evaporative cooling efficiency of an organism (Robertshaw 1985). In humans, the greater proportion of evaporative cooling occurs through sweating, and very little evaporative heat loss occurs through respiration due to the small nasal chamber and the associated reduction in the area of mucous membrane available for evaporation to occur.
Thermoregulatory Adaptations and Behaviors
A variety of adaptations, physiological devices, and behaviours have been noted to address the thermoregulatory priorities of mammals. All are important in the understanding of human thermoregulation and its evolutionary history. The important mechanisms that must be discussed when addressing the issue of human thermoregulation are body proportions, metabolic regulation, vascular compensation, body fat, behavioural modification or cultural practices, body hair, and sweating.
The adaptation of body proportions to environmental conditions (ecogeographical patterning) is a well-established principle of evolution. This premise is based on early work by Bergmann (1847), who noted that populations of the same homeothermic species will differ in average weight with those in colder climates heavier than those in warmer climates, and Allen (1877), who noted that populations of the same homeothermic species will differ in average limb length with those in colder climates having shorter limbs than those in warmer climates.
These rules are special cases of the general relationship between surface area, body mass, and ambient environmental temperature. Fourier’s Law of Heat Conduction indicates that the rate of heat loss from the core of an animal is directly proportional to the surface area available for heat conduction to the environment and inversely proportional to the distance that is needed to be travelled from the core to the surface. If an object increases in size allometrically the volume will increase at a much greater rate than the surface area, which means that the surface area to volume ratio will decrease.
Since heat loss is directly proportional to the surface area and inversely proportional to the volume and increase in body size will decrease the rate of heat loss (as a proportion of body heat) through convection across the skin/environment gradient (Ruff 1991). This relationship has been studied in many living animals (including humans) and is particularly robust (Riesenfeld 1981; Holliday 1995). This relationship also means that with increasing body size, the removal of body heat becomes more important than the retention of body heat, because less and less heat will be lost with increasing size. This is an important implication for human evolution because humans and their hominid ancestors are relatively large mammals.
Metabolic regulation of body heat is an important aspect of the human physiological thermoregulatory response. In cold environments the metabolism will speed up, causing an increase in metabolic thermogenesis, and conversely, in warmer environments the metabolism will slow down, causing a decrease in thermogenesis. The level and efficiency of the metabolism are regulated by several hormonal and neuro-regulatory mechanisms. The thyroid gland can both increase and decrease the metabolic thermogenesis by regulating the release of thyroxine into the body, producing both short-term and long-term changes in metabolic thermogenesis. In addition, the release of adrenaline and noradrenaline from the adrenal medulla can also cause increased thermogenesis. The neuro-regulatory system also can initiate reflex shivering in muscle tissue, which is the activation of muscle tissue in continuous transient contraction-relaxation cycles that produces waste heat (Hanna & Brown 1983; Senay et al. 1976; Bass et al. 1955; Lind & Bass 1963).
The human vascular system has developed reactions to both heat stress and cold stress. The skin has a system of thermal receptors that perceive the temperature of the skin and send signals carrying this information to the autonomous nervous system. When the body perceives increased heat loss through the skin, vasoconstriction of the peripheral vascular system occurs to decrease the blood flow (which carries heat from the core to the surface). In humans, this vasoconstriction can reduce heat loss by 1/6 to 1/3 depending on the individual and the acclimation of the individual to cold stress.
When the body perceives a need for increased heat loss, vasodilation occurs, with increased blood flow to the peripheral vascular system. This vasodilation increases the rate of heat transfer from the core to the surface and is also an important feature involved with sweating. This vascular compensation has associated changes in the internal vascular system, and in vasoconstriction, increased arterial pressure occurs due to the reduced volume of vessels with the maintained blood volume. In vasodilation, constriction of the internal vessels occurs that can increase an individual’s susceptibility to postural hypotension and can lead to transient cerebral ischemia (Senay et al. 1976; Bass et al. 1955; Lind & Bass 1963).
Body fat is located under the dermis in a subcutaneous layer. This fat can have several functions in mammals that may or may not be related to thermoregulation. Adipose tissue can act as a source of stored energy, thermal insulation, and a source of chemically produced heat. In some water mammals, an insulating layer of blubber has developed that prevents excessive heat loss from the body to the environment, but the functional significance of the fat layer in humans as an insulating device is negligible. In humans, the fat layer acts as a source of stored energy, and with regard to thermoregulation, acts as a source of thermal heat through lipolysis. When the fatty acids of this tissue are oxidized to produce energy, a large quantity of heat is produced. The development of a thick layer of subcutaneous fat may be an adaptation to the loss of body hair and the potential cold stress that the human ancestor would have experienced, or may simply be a feature of the behavioural capacity of humans to get access to food.
Behavioural modifications can include any behavioural feature that can mitigate heat loss or produce heat in a cold environment or mitigate heat gain or release heat in a warmer environment. These practices can include simply staying out of the sun during the hottest part of the day, seeking shade when moving through a hot environment during the day, or building nests or seeking sheltered areas in cold environments (Wheeler 1994; Napier & Napier 1985). Cultural practices might include things such as the use of fire, the production of clothing, the building of shelters, or other human cultural practices (Kushlan 1985). These cultural and behavioural features are important ways in which humans and their ancestors dealt with – or could have dealt with – heat or cold stress, but these features can be very variable and their effectiveness and presence are hard to identify among fossil species.
Body hair is one of the most important features of the mammal thermoregulatory system. Hair can act as important heat retention and heat prevention device in mammals. By trapping a layer of dead air against the skin, a layer of hair can act as extremely efficient insulation, reducing the rate of convective heat loss to the environment. However, this exact same system acts as a way to prevent heat gain from the environment by the same principle; by using this layer of dead air to reduce the rate of convective heat gain from the environment to the skin. Besides insulation, the layer of hair on mammals is important in reducing the radiation from direct and indirect sunlight, and can thus act to reduce heat gain from the environment in two ways.
Sweating is one of the thermoregulatory traits that set humans apart from most other primates. Sweating acts as a heat loss mechanism through evaporation. The sweat on the surface of the skin is mostly water and has high specific heat. Heat is removed from the skin by conduction to the sweat until the sweat is either evaporated or sloughed off. Sweating is most effective if the sweat is actually evaporated since it is removing more heat per unit of sweat being produced. Sweating is highly adaptive in modern humans and is associated with the development of eccrine glands throughout the skin, the reduction of body hair, and vascular compensation (Montagna 1985).
Eccrine glands are specialized sweat glands that produce large quantities of sweat that is mostly water and has coevolved with the loss of body hair to create a more effective evaporative heat loss mechanism. Vasodilation increases the blood flow from the core to the periphery while increasing the rate of heat loss to the skin, which is then removed through the evaporation of sweat (Ebling 1985). While all the mechanisms for controlling body temperature that have been mentioned are important when considering the evolutionary perspective of human thermoregulation, the loss of body hair and the coincident evolution of eccrine glands throughout the body deserve more detailed description.
The Development and Structure of Hair
A major difference between humans and other primates is the great reduction of body hair in humans relative to other primates. The question of this difference has often been asked in the context of the loss of human hair, but this is not strictly true. Modern humans are not hairless in the sense of being glabrous, but rather have had a significant diminution of body hair length and thickness relative to other primates. The only hair structure that has been truly lost in the human condition is the vibrissae, which are the sensory whiskers that are common in most mammals (Van Horn 1970). Other categories of hair form are seen commonly in humans and are no different developmentally from other African apes. Human hair can be distinguished into three main categories of hair that include lanugo, vellus and terminal hair types. Lanugo hair is the first hair produced by the developing hair follicles during prenatal development. This hair forms subdermally by the third or fourth month of fetal development, and by the fifth month, the hair structure has appeared on the external surface of the skin of the fetus. This hair tends to belong, unpigmented and very fine, and is generally shed by the eighth month of fetal development, though it may persist until a month or more after birth (Gray 1974).
The first hair that is produced during the post-natal life of an individual is vellus hair. The vellus hair is generally short, unpigmented, very fine, and unmedullated. This is the hair that primarily covers the human body in most individuals, the hair that covers the so-called “hairless” parts of the skin, such as the forehead or the nose. This hair may also replace terminal hair in individuals with androgenic alopecia, the typical form of baldness that is seen in many human beings. Terminal hair tends to belong, coarse, pigmented, and medullated. Among humans, terminal hair covers the scalp, axillae, pubic area, and the face and chest of some males. Hair follicles are capable of producing either vellus or terminal hair, and under hormonal stimulation can switch from producing one to the other (e.g. during puberty, when vellus hair in the pubic region begins to be replaced by terminal hair, or in baldness when vellus hair replaces terminal hair) (Montagna 1976).
The embryogenesis of hair follicles and hair is an important consideration in the discussion of the loss of the human pelage, as it is during this development that the apocrine gland system develops, the implication of which will be discussed in detail later. The development of the hair follicle begins with the establishment of the dermal papilla (DP). The DP is a group of specialized dermal fibroblast cells that are derived from the mesoderm and begin to aggregate below the epidermal layer. In the epidermal layer, an epidermal plug forms above the DP and grows into the dermis toward the DP as the cells proliferate. The plug begins to differentiate into three distinct cell buds as the proliferation of the cells progress. The cell bud closest to the epidermal layer may develop into an apocrine gland, or will gradually regress as the hair follicle matures.
The cell bud in the middle gradually develops into the sebaceous gland, while the final cell bud below forms what is called the “bulge”. This bulge on the hair follicle is the site of attachment for the arrector pili muscle (the muscle that pulls the hair perpendicular to the skin when one gets “goosebumps”), which develops separately from the hair follicle and eventually attaches to this site. As the epidermal plug penetrates further into the dermis, mesodermal cells begin to surround the plug and develop into the fibrous follicular sheath that surrounds the epidermal cells. As the epidermal plug is differentiated and migrates into the dermis, the DP develops into a structure of rounded cells that contain organelles vital for product synthesis, and its cells becomes non-proliferative. The DP cells then communicate with the epidermal plug, effecting differentiation into concentric layers that will eventually become the hair fibre and the inner and outer root sheath encasing the hair. These cells begin to keratinize and die higher up in the layers while the deeper cells continue to proliferate and force the keratinized hair fibre to extrude from the surface of the skin (Holbrook 1991).
The growth of the hair itself within the follicle follows a cyclical pattern. There are three main stages of human hair growth: anagen, catagen, and telogen. The anagen phase is further subdivided into proanagen, mesanagen, and metanagen. Anagen is the active growth phase of the hair and the state in which any individual hair will spend most of its life cycle. In the proanagen phase, the initiation of growth with RNA and DNA synthesis in a follicle occurs, which then quickly progresses through mesanagen to metanagen with the resulting maximum hair length and thickness achieved. In this mature state of proliferation and differentiation, the hair follicle is made up of eight concentric layers, and melanogenesis will occur within pigmented hair follicles.
The anagen phase is followed by catagen, which is a period of controlled regression of the hair follicle where the dermal papilla migrates upward and the growth of hair is reduced. This results in a weaker fit of the hair in the root sheath and a thinner hair. Finally, the hair follicle will enter the telogen phase, a resting state where little or no growth occurs, and where the hair will fall out or can easily be pulled out. At this stage a new dermal papilla will begin forming in the dermis and a new hair will begin to grow, forcing the old hair out of the skin if it has not already been removed (Moretti et al. 1976). In many mammals, adjacent hairs go through these cycles in waves (the loss of gain of the “coat” in particular climatic seasons), but in humans, the hair proceeds through these stages independently of any other follicle, and during any one moment approximately 90% scalp hair will be in the anagen phase, and 10% will be in the telogen phase (Kligman 1988).
The Sweating Mechanism in Humans
The sweating mechanism of modern humans is the single most important thermoregulatory device available to reduce the heat load on the body and likely has coevolved with the loss of body hair in the human lineage. Sweating is a thermoregulatory mechanism of modern humans that effectively removes body heat through evaporation. It becomes extremely effective in the absence of heavy body hair, and actually can be maladaptive in the presence of heavy hair cover. The structure and function of the human sweat glands share some common aspects with the African Apes (though much less with other closely related primates such as the orangutan), and also have some relatively unique features. As in most primates, the human system of surface excretory glands includes three structural forms: sebaceous, apocrine, and eccrine glands. Sebaceous glands are tied to every hair follicle and produce small amounts of oils and fluids that maintain the suppleness of skin and hair fibres.
These glands are important in the production of odours at the axillae and pubic regions (most likely tied to sexual selection), but have little to no significance in terms of sweating prodigiousness or efficiency. Apocrine glands also develop in association with the hair follicle and secrete by the rupture of cell apices at their luminal margins, releasing fluids containing cellular components. These cellular components are broken down in the bacteria of the microenvironment of the axillary areas of the body, producing the body odours in humans. Eccrine glands develop independently from the hair follicles and produce large quantities of sweat that is composed primarily of water and mineral salts. Sweat from these glands does not produce odour and is the primary cooling mechanism of modern humans through evaporative cooling (Ebling 1985; Montagna 1985).
The distribution of these glands along the body follows a very regular pattern. Sebaceous glands are found in association with all or nearly all hair follicles and have no known functional significance in thermoregulation. Apocrine glands are found in the axillary regions (the pubis, the perianal region, and the axillae) in humans, whereas in most non-human primates (excluding gorillas and chimpanzees) they are found throughout the entire body. It is important to note that in the human fetus apocrine glands begin to form all over the body in association with hair follicles, but are mostly resorbed into the body during development.
Eccrine glands are found over the entire surface of the body, in both hairy and non-hairy areas, and have no developmental tie to individual hair follicles. In most non-human primates (again excluding gorillas and chimpanzees) eccrine glands are only found on surfaces used in locomotion (the soles of the hands and feet, and among the dermatoglyphics found on places of high friction, like the tails of prehensile species or the knuckle pads of knuckle-walkers. There are two phylogenetically distinguishable types of eccrine glands, those found in the friction areas of most species of primates and many other mammals, and the ones found in humans, chimpanzees, and gorillas throughout the entirety of their body. In humans, the eccrine glands of the volar surfaces of the hands and feet begin to develop around three-and-a-half months of fetal development, and the remaining eccrine glands begin forming separately around five-and-a-half months (Montagna 1985; Robertshaw 1985).
The sweat glands themselves also exude their secretions at different rates and under different pressures. For example, the sebaceous glands continuously produce secretions to maintain the hair and skin surface, the apocrine glands produce continuously in the axillary regions, the eccrine glands in the friction surfaces also produce relatively constantly at a low background rate, and the eccrine glands spread throughout the body can either not produce secretions over extended periods of time, or produce vast quantities (more than twice as much as any other mammal) of sweat over time. There are two ways in which these secretions are controlled: adrenergic and cholinergic stimulation. The apocrine glands and the eccrine glands of the friction surfaces are controlled by adrenergic sympathetic nerves and are stimulated by emotional stress (associated with higher production of hormones like adrenaline), while the eccrine glands of the rest of the body are controlled by cholinergic nerves, and can be stimulated by heat stress. This system is relatively constant in non-human primates (Robertshaw 1985).
The functional aspects of the various glands are also different for different glands. The sebaceous glands – as mentioned previously – act as lubricants for the skin and hair, maintaining moisture content and prevent the skin and hair from drying out and cracking. The apocrine glands in humans (and gorillas and chimpanzees) seem to have a sexual function and produce odours. This occurs through the combination of the sebaceous, apocrine, and eccrine glands. In the axillary areas, the sebaceous and apocrine glands produce a constant stream of secretions of high cellular content. When the eccrine glands are stimulated to produce sweat, the sweat mixes with the secretions of the sebaceous and apocrine glands and spreads throughout the axillary area.
In the moist microenvironment of these areas, bacteria are abundant on the skin and on the hair, and when given access to the organically saturated sweat, begins to break down the cellular components, with body odour as the result of this process. The sweat excretions themselves are odourless. This mechanism only occurs in humans and the African Apes and does not occur in orangutans or other non-human primates (which usually have a scent-producing organ in a sternal pit above the manubrium). Since this mechanism only begins working after puberty (apocrine glands are primarily non-functional in prepubescent humans) it seems likely that this is a product of sexual selection and has a function in mate selection rather than any sort of friction-reducing function (Montagna 1985; Hanson & Montagna 1962; Perkins & Machida 1967). The eccrine glands that cover most of the skin of humans seem to have one single function, thermoregulation through evaporative heat loss, and this function is apparent only in humans. In chimpanzees and gorillas, the eccrine glands on the body are nonfunctional and do not react to thermal stress.
Evaporative heat loss through the use of the eccrine gland system in humans is extremely effective at removing unwanted body heat. Evaporative heat loss occurs through the secretion of sweat primarily made of water, the heat exchange from the skin (that is artificially maintaining a larger proportion of heat due to the highly developed vascular system that moves heated blood from the body core to the surface at high rates) into the sweat, and the evaporation of the sweat as it is either sloughed off or evaporated into the air.
Effective sweating requires as little hair cover as possible, as it needs air contact (particularly moving air) over the skin to remove the heated sweat. In an individual with a prodigious sweating mechanism and dense hair cover, the heated sweat will generally be retained by the hair cover and actually begin to act as insulation preventing heat loss, leading to hyperthermia. Non-evaporative conduction becomes less and less effective with increased body size (due to the decreasing surface area to body size ratio) and with increased ambient temperature (due to a lower temperature difference between the environment and the organism) making evaporative heat loss through sweating an extremely important adaptation in the relatively large-bodied ancestors of humans in the warm climate of Africa (Robertshaw 1985; Schwartz and Rosenblum 1981).
Although hair can impede heat loss through sweating, it is important in thermoregulation for maintaining body heat in either colder environments or at night and for the reflectance of solar radiation away from the body. Dense hair cover is very efficient for both of these purposes, and it is an important question to ask why the selection for increased sweating efficiency for heat loss would be more important than the selection for the heat retention mechanism of hair cover and the heat prevention mechanism of solar radiation reflectance. Dense hair cover is an effective heat retention device, but only in smaller animals. As animals in an increase in size, the effectiveness of hair cover decreases. This is due to the reduced ratio of skin area to volume as mass increases.
As body size increases, the amount of metabolically derived heat increases dramatically, but the ability of the organism to effectively lose this heat is retarded by the decreased ratio of surface volume. Thus, the percentage of heat lost to the environment by conduction decreases, simply because the organism loses the ability to lose heat as size increases. This means that the hair cover is less and less evolutionary meaningful for the retention of body heat. The corollary of this axiom is that as body size increases, and the metabolic heat load increases, there is an increased need for mechanisms to remove heat in hotter environments or in periods of high metabolic heat production. So as body size increases, dense hair becomes less and less effective at retaining body heat, and more and more maladaptive for removing body heat (Schwartz & Rosenblum 1981; Robertshaw 1985).
The obvious solution to this situation is decreased body hair with increasing body size, which is exactly what is seen in anthropoids. When the number of hair follicles present in species per unit of area is compared with body size, all primates (including humans) fit along a regular log-linear regression line, along which the density of hair per unit of area decreases as body size increases. Species like chimpanzees and gorillas have relatively fewer hair follicles per unit area of skin compared to the smaller monkeys. Humans fall along this line and have a relative hair density almost the same as seen in chimpanzees, gorillas and orangutans. The difference between the thick pelage of the Great Apes and humans is not in terms of the density of hair but in its length and thickness and the production of vellus hair in most humans to the exclusion of terminal hair on the body. Humans are not “hairless”, but are merely covered by thinner, smaller and unpigmented hair (Schwartz & Rosenblum 1981; Schultz 1931).
The effect of solar radiation reflectance on a selection pressure to maintain body hair has been considered many times, but a satisfactory answer has not been explicated. It is clear that thick body hair protects most mammals by reflecting solar radiation, and some theories have tried to account for this (Wheeler 1991a), but it seems likely that it is more a result of competing pressures being stronger, leading to the reduction of hair even in the face of this pressure to maintain body hair for reflective purposes. Attempts to quantify the relative importance of reflective hair in the question of human hair loss may be a false problem, as we know that humans do have little hair, and it is generally agreed that our ancestors at some point did have a heavier coat of hair. It may be less important to ask why hair loss developed in the face of this particular pressure to maintain it but rather what other pressures could have caused the hair to be selected against.
Expectations of a Putative Human Ancestral State
The preceding description of the modern human thermoregulatory system and the structure and function of its individual parts allows the reconstruction of an expected ancestral state when one compares the differences between humans and their closely related primate species. It is generally assumed that shared descent is more reasonable than paralleled development, and thus from this assumption of parsimony (keeping in mind the chance of parallelism inherent in closely related species inhabiting similar environments or facing similar selection pressures) several points clearly present themselves.
The presence of phylogenetically distinct and functionally and structurally differing sweat glands within humans and several closely related species indicate that either the distribution of eccrine glands (and the associated reduction of apocrine glands throughout the body) can be traced back to the common ancestor of chimpanzees, gorillas, and humans, that it can be traced back to the common ancestor of chimpanzees and humans with parallel development in Gorilla (or common ancestry for humans and gorillas with separate origin in Pan depending on the phylogenetic tree used), or that it has a separate origin in all three lineages.
Through parsimony, the least likely scenario would be the complete independent formation of the eccrine distribution, but it should be noted that in a species of tree shrew, an eccrine system has developed independently; and therefore independent origin is not at all impossible. Proceeding to a more parsimonious answer, the shared derived state of two lineages and the independent origin in one species, it is unclear if this is – in reality – more likely than complete three-way independent origin, as if the selective pressures under whose aegis this trait would develop was common to all lineages and developed independently in at least two lineages within a relatively short evolutionary timeframe, it may be no more likely for two origins versus three, though it is more parsimonious. The most parsimonious explanation is the single origin of the trait and the shared derived status of the eccrine distribution in the three African lineages. Parsimony leads to the most logical choice in terms of probabilities within a sterile mathematical phylogeny but does not always provide the most likely scenario, which must be determined from the whole of available evidence.
The reduction of body hair thickness and length in humans versus chimpanzees and gorillas indicates that it is most parsimonious that hair reduction occurred in the human lineage after the Homo-Pan split. The relative density of hair follicles per unit of skin is approximately the same in humans, chimpanzees, and gorillas. This is not necessarily surprising since with increasing body size and therefore increasing skin surface area, one would expect a reduction in density scaled to body size. When a species gains size it does not grow extra limbs, so why would it grow extra hair follicles? When put in the perspective of a human ancestral state, it is therefore likely that any ancestor of the three lineages back through the common ancestor of all three should have had a similar consistency in relative hair density, and any difference in perceived pelage density would be a result of individual hair thickness and length.
From the description of the growth and development of hair in humans, it is clear that differences in thickness and length are the result of different growth rates, differing lengths of the growth cycle, and possibly different proportions of time spent in each phase of the hair life cycle. Since a wide variation exists in the perceived body hair in modern humans (as well as between individuals among the chimpanzee and gorilla population), it seems likely that the relative “hairlessness” or “hairiness” of populations is extremely malleable under evolutionary pressures. Reduction in human body hair did not require a complicated, dangerous, or unlikely change to the genetic controls for hair growth, but simply normal natural selection on variation within the traits that control hair growth. Therefore, while the most parsimonious explanation of human body hair reduction is the occurrence after the Homo-Pan split, it is not inconceivable that it could have happened earlier with an associated “reversal” in one or both of the Pan or Gorilla lineages.
The placement and function of apocrine glands in the axillae and pubis occurs within humans, chimpanzees and gorillas. However, this does not occur in other closely related anthropoids such as the orangutan. Putting aside the question of the functional significance of this arrangement, there is a clear distinction between the pattern of apocrine gland distribution in the Homo-Pan-Gorilla clade and all other closely related anthropoids. This indicates that the ancestral state of the human lineage was likely the same and that whatever adaptation or simple neutrally selected change that occurred in the past occurred prior to the split of these three lineages. While of very little thermoregulatory importance, this common functional and structural distribution is important in relation to the selection against general apocrine gland distribution throughout the body and associated radiation of eccrine glands throughout these areas. Any theory that invokes a thermoregulatory explanation needs to address this issue as well as anything pertaining to the particular theory.
The distribution of a thick subcutaneous fat layer in modern humans is another “unique” feature in relation to closely related non-human primates and should be considered in any theory that invokes a thermoregulatory explanation. However, this is hard to distinguish as either a true problem or a false one, as humans do not have any more points of fat production than other primates, but rather have more fat developed from these individual centers. This can be seen as simply an effect of the ability of humans to procure food sources much greater than their needs with relative ease. In this manner, the question of subcutaneous fat may be a red herring with regards to thermoregulation. Nevertheless, the important physiological impact of this fat layer would still impact any selective pressures for changes in the features related to thermoregulation, and as such, the fat layer and its features should at least be accountable under any particular theory.
Theories of Human Evolution and Thermoregulation
All large variety of hypotheses have been proposed that inherently deal with thermoregulation as either a first principle or as a causally effected change due to some development. These arguments range from just-so stories to parsimonious explications to non-falsifiable single cause arguments used to explain a wide range of features. Each theory will be examined in terms of how well it deals with the points above about what would be the expectation(s) of an ancestral state, the validity of the explanations for various thermoregulatory features present in the human condition, and a general assessment of the relatively reasonable nature of the hypotheses. Examination of each theory is limited to the points relevant to thermoregulation.
Aquatic Ape Hypothesis: The Aquatic Ape Hypothesis belongs to the category of theories that Langdon calls “umbrella hypotheses”, meaning that such theories attempt to explain everything about the modern human condition and human evolution through primary cause explanations (Langdon 1997). This theory has its roots in an article published in New Scientist in 1960 by marine biologist Alister Hardy. Elaine Morgan developed the theory through the publication of several popular feminist books and essays in the 1970s, 1980s and 1990s (Morgan 1972, 1982, 1990). The basic premise of this theory is that a human ancestor at some stage went through a semi-aquatic phase during which the reduction of body hair, the development of subcutaneous fat, the development of eccrine glands, the reduction in apocrine glands, and about every other difference between modern humans and extant primates developed. This critique of this hypothesis will focus on the specific adaptations related to thermoregulation.
In general, the logic lines proceed in the following fashion. The thermoregulatory problems of a water environment are much different from that of a terrestrial environment, leading to a series of unique adaptive solutions in the human lineage with respect to closely related extant primate species. The reduction of body hair in humans occurred as a consequence of its uselessness for insulation in the water environment. This is a problematic statement, especially when placed in the context of the aquatic ape as “semi-aquatic” since hair loss in water mammals occurs as a result of a full-time aquatic existence.
The hair would be an effective insulator as long as the air was trapped in the hair; it would only be as a consequence of extended periods in water that the hair would become completely soaked and useless for insulation. In fact, most semi-aquatic mammals (i.e. spend time on land as well as in the water) retain their body hair and have developed extremely dense fur cover. Full-time aquatic mammals such as cetaceans and extremely large-bodied semi-aquatics have lost or had greatly reduced body hair, but all these animals are large-bodied, and similar sized terrestrial mammals often lose their body hair as well.
The presence of a subcutaneous fat layer in humans is explained as both an adaptation for insulation and for buoyancy. However, while this is functionally correct to some extent in humans, this idea lacks explanatory power in the face of equally likely or even more likely explanations that have been put forth to explain the same phenomenon. It is highly unlikely that in a fit individual the fat layer acts significantly in creating buoyancy as to be more or less selectively adaptive. As well, the thickness of the fat layer in a fit individual makes the utility of the fat layer in insulating from the high rate of convective heat loss with the water environment questionable. At best, the subcutaneous fat layer fits the aquatic model but does not provide independent evidence for it, as there are equally likely explanations available.
The development of eccrine glands is explained by the aquatic model as a mechanism for removing excess salt from the body in an environment where salt would be plentiful and over consumed (a marine environment). This explanation is especially problematic. There are very few environments where both freshwater and salt are independently available in large quantities. Freshwater animals must conserve salt while marine animals must excrete excess salt from their bodies while retaining water. Dehydration is a marine mammal is as dangerous as in a terrestrial mammal. Morgan speculates that humans once secreted a hypertonic sweat relative to blood plasma in order to remove salt while retaining water (Morgan 1990).
However, the aquatic model neglects to explain why human sweat is now hypotonic with the active recovery of salt in the eccrine gland before sweat is secreted, in essence removing large amounts of water with relatively little salt content. This same quandary for the aquatic hypothesis is seen in the urine of humans, that is much more dilute than other mammals, meaning even more water is lost through the urinary system. Some adherents to the aquatic model have claimed that humans must have inhabited both marine and freshwater habitats simultaneously or successively (Verhaegen 1985), but this would now effectively involve the movement between three environments, making it even more unparsimonious when the terrestrial-based theories can explain the same phenomena with only one major environmental milieu.
The function and distribution of eccrine glands are completely contradictory to the aquatic model. Attempts to fit the data to the model by appealing to unexplained and unproven evolutionary changes in the human line since the putative aquatic phase are not reasonable and not robust in any objective way of looking at the problem. The development of eccrine glands in humans for the removal of excess salt also is problematic in light of the eccrine distribution in chimpanzees and gorillas.
There is no argument for an aquatic phase in these lineages, and yet they show an adaptation that supposedly developed in the human lineage for the express purpose of dealing with a problem unique to a marine existence. In addition, Morgan claimed that humans do not have an innate “salt hunger”, which would be expected in a species that developed in an environment where salt is in oversupply. However, this is clearly not the case, and humans show an extremely high sensitivity to declining salt intake (Denton 1982). The aquatic model does not fit more parsimoniously or reasonably with the available data than any theory explaining these features on the basis of the thermoregulatory advantage of sweating.
The aquatic model explains the reduction in apocrine glands over the body as a function of reduced sexual selection. The logic of this argument follows that apocrine glands secrete pheromones that would have been washed off in the water, and thus would have lost their significance in an evolutionary perspective. This explanation does not fit the actual distribution and function of apocrine glands in humans and other primates. It is true that humans have a reduced distribution and number of apocrine glands over the body; however, this trait is held in common with chimpanzees and gorillas.
In addition, it is in these exact species where apocrine glands may have the function of sexual signals. Most other primates – including other closely related anthropoids such as orangutans – have specialized structures to release pheromones, such as sternal pits above the manubrium, rather than generalized apocrine glands distributed over the body that act as pheromone producing machines. In yet another instance the aquatic model does not realistically address the physiological realities related to structures involved in thermoregulation. Regardless of the parsimonious nature – or lack thereof – of other theories, the aquatic ape hypothesis does not in any shape or form adequately explain these features of the human thermoregulatory suite of mechanisms.
Bipedality: In the 1980s, a hypothesis was developed through a series of articles by Peter Wheeler that attempted to explain the human sweating mechanism and hairlessness through the first cause of bipedality (Wheeler 1984, 1985, 1991a, 1991b, 1992, 1994). Wheeler argued that a bipedal hominid would have a significant thermoregulatory advantage over a quadrupedal hominid (Wheeler applied the term “hominid” improperly to pre-hominid quadrupeds as well as hominids sensu stricto) due to the decreased exposure to direct and indirect solar radiation and would increase skin surface area to winds, which would increase the selective value of the sweating mechanism. This thermoregulatory advantage was argued to be great enough for natural selection to drive hominid evolution towards bipedalism.
The loss of body hair and the development of an effective evaporative sweating mechanism were seen as adaptations that became advantageous only after the change from quadrupedalism to bipedalism. The primary problem with discussing this model is that Wheeler documented the thermoregulatory benefits of bipedalism to an already bipedal hominid, and then the thermoregulatory benefits were used as the cause of bipedality itself, with associated thermoregulatory mechanisms contingent upon bipedalism.
This logic, like all circular reasoning, is not clearly falsifiable in terms of an explanatory method, but only in terms of the truth of the premise. In this case, the premise (the thermoregulatory advantage of bipedalism through the reduction in direct solar radiation) is generally accepted, and therefore the hypothesis cannot be falsified in terms of the logic of the relationship. However, the argument for the cause of bipedality is not the purpose of this chapter, and this analysis will focus on the statement that bipedalism is a prerequisite for hair loss in the human lineage.
It is Wheeler’s proposition that bipedalism is an adaptation to a savanna environment where the thermoregulatory advantage of bipedalism would lead to its evolution. After this change, it would be advantageous for the loss of body hair. Wheeler further claimed that in a naked biped there would be a reduction in the water requirements during normal metabolic activity on the African savanna. Since quadrupedalism offers no such advantage to an individual with reduced hair cover, bipedalism was a prerequisite for hair loss since it gave a thermoregulatory advantage that would have made hair loss selectively adaptive. However, there are several problems with the analysis and interpretation of data presented by Wheeler.
Wheeler’s calculations of heat load on a bipedal versus a quadrupedal animal are obfuscated by the use of the metabolic load of an inert animal rather than that of an animal in motion (Chaplin et al. 1994). This way of determining the metabolic component of heat stress in an animal effectively increased the hypothetical advantage of bipedalism in a hot environment by making the main determinant of heat stress environmental heat load. This means that any behavioural act would make no difference in the heat stress of an animal, leaving any hypothesis regarding what the selective thermoregulatory advantage of bipedalism in terms of behaviour strongly supported.
However, maximal exercise heat loads created by high metabolic output in medium-sized mammals (>10 kg) can reach up to ten times the level of the environmental heat load (Taylor 1977). This means that the relative advantage in terms of foraging time available to an animal through bipedal locomotion over quadrupedal locomotion is greatly reduced when compared to calculations based on an inert metabolism. While this greatly reduces the thermoregulatory advantage of bipedalism compared to what was claimed, there is still an advantage in the reduction of environmental thermal stress.
Bipedalism was claimed as a necessary pre-adaptation for the loss of body hair in the human lineage. However, the question was approached from the perspective of the relative advantage of bipedalism versus quadrupedalism for an animal with reduced hair cover. Since the calculations showed that a naked biped was more efficient than a naked quadruped, bipedalism is claimed as a necessary step prior to denudation. However, Wheeler neglected to address the question of whether a naked biped would have an advantage over a haired biped in his calculations. This question is implicitly answered with the comparison of thermoregulatory efficiency of a haired biped versus a haired quadruped and of a naked biped versus a haired quadruped.
This comparison is faulty because Wheeler assumes a maximum heat dissipation of 100 W/m2 for a haired primate versus a maximum of 500 W/m2 for a modern human. The human level of heat dissipation occurs through the use of evaporative sweating, which would have developed in concert with the loss of body hair, and thus cannot be used as the level of heat dissipation that would make developing bipedality advantageous. Also, the 100 W/m2 level is too low, since it is a measure from forest-dwelling primates, and there is a savanna adapted primate that has a heat dissipation of over 200 W/m2 (Mahoney 1980). A scenario that makes bipedalism a necessary pre-adaptation for the loss of body hair means that bipedalism would have occurred in the absence of the development of the sweating mechanism, and thus the heat dissipation of this proto-biped would not have had the heat dissipative capabilities of a modern human.
When this factor is accounted for, the putative greater heat load capacity of a naked skin of a newly developed biped over denser body hair is negated. Amaral (1996) showed that the thermal stress on a naked biped is up to three times greater at higher temperatures than on a hair-covered skin. In fact, this is exactly the reason that other savanna primates have a dense coat of fur that is even more developed than forest-dwelling primates do. It is only the fully developed modern human sweating capacity that effectively compensates for this, making the development of an efficient sweating mechanism a necessary pre-adaptation for hair loss. Amaral also pointed out that the advantage of bipedal stance in placing the body in the path of wind for evaporative heat loss is only advantageous if the temperature of the wind is lower than that of the body temperature of the animal. If the ambient air temperature is higher than body temperature, the exposure to wind will actually increase the heat load, even more so in hairless individuals (Cabot Briggs 1975).
This same problem is inherent in Wheeler’s assertion that bipedalism is more water-efficient on the hot savanna environment if one has lost body hair cover. What Wheeler’s calculations show is that this is true only if the air temperature is lower than body temperature. In the hot savanna environment, where ambient air temperature will commonly exceed the 35°C of body temperature, the loss of hair provides no advantage over dense body hair. These points do not rule out the possible thermoregulatory advantages of bipedalism (however, claiming that because it was more thermoregulatory efficient it would develop is deterministic and similar thinking would mean that all other mammals in hot environments should be bipedal as well), but they do make the assertion that bipedalism is necessary for the loss of body hair unreasonable. If bipedalism develops as a consequence of its thermoregulatory advantage in hot climates with steep environmental heat stress, the loss of body hair subsequent to this should not happen due to the relative disadvantage of hair loss under steep environmental heat stress.
Sexual Selection: The idea that the loss of body hair in the human lineage was a result of sexual selection is a very old one. Darwin first presented the idea in 1871 (though a footnote in the text attributes the idea – inspiration? – to Reverend T.R. Stebbing) in a text on sexual selection, which Darwin believe worked in concert with natural selection in the evolution of species. Darwin’s original reasoning can be considered faulty in retrospect (“no one supposes that the nakedness of the skin is of any direct advantage to man; his body hair, therefore, cannot have been divested of hair through natural selection”), but some of his observations are still considered to hold true. For example, the facial hair of men has no functional or adaptive significance that can be explicated. It is a plausible explanation for the differences between male and female facial hair is sexual selection, or possibly the difference in body hair in males and females. However, invoking sexual selection as the cause of the loss of body hair in humans encounters several hurdles.
The presence of an effective sweating mechanism for evaporative heat loss is greatly dependent on the loss of body hair in humans. If sexual selection is ultimately responsible for the loss of body hair in humans, the sweating mechanism would have to have been selected for by a separate mechanism, even though the two features would have had to develop in concert for sweating to remain effective. This is not in any way impossible, as it might be argued that the sexual selection leading to hair loss would have allowed the selection for an efficient sweating mechanism.
In this scenario, sexual selection would not even have to be the main driving force of the loss of body hair; it would at the very least only be required to initiate the reduction in body hair, at which point natural selection for the thermoregulatory efficiency of the sweating mechanism might drive the denudation of the human lineage. This is not unreasonable and therefore is an open option even when discussing the selective benefits of sweating as the driving force in the loss of body hair in humans. However, while it is an option that cannot be negated, the invoking of two selective pressures to explain a single phenomenon when one can explain it just as well makes the explanation less parsimonious, though not necessarily unlikely or unreasonable.
Vestiary Hypothesis: The vestiary hypothesis of human hair reduction maintains that human culture is responsible for the loss of human hair. The theory states that with the advent of increased brain size and increased intelligence, humans began to use fire and make clothing, making the selective maintenance of hair for the retention of heat redundant. Since there is no fossil evidence for hair, supporters say there is no reason to assume that the reduction in human hair happened long in the past. However, this idea ignores the basic properties of thermoregulation and does not explain why, even if the hair was redundant, it would be selected against in humans. This idea also does not address the issue of the eccrine and apocrine distribution of the Great Apes, which mirrors that of modern humans (Hamilton 1973; Kushlan 1985). Many researchers do not seriously accept this theory, as it has not been well developed and ignores many of the physiological features involved in the loss of body hair and the need to explain those features.
Hunting Hypothesis: In the 1960s most models of human evolution focused on the importance and evolutionary significance of hunting. Ardrey (1976) put a name to the idea of hunting as a causal factor in the loss of human hair, and laid out the arguments that had been floating about since at least the early 1960s. The line of reasoning for the loss of body hair and the development of an evaporative sweating mechanism – in general – went in the following manner. A change in habitats through climatic shifts led to a shrinking ecological niche for the ancestors of humans.
The human line left the forest and took to the savanna to exploit new environmental opportunities. This change in ecology led to a change in diet that proceeded to add insects, eggs, and small animals to the nuts and fruit they previously had been supported by in the forest environment. This dietary change progressed until they were hunting mammals. Hunting required a bipedal stance in order the use of the hands for tools and weapons, and the thermoregulatory stresses of the hunt required the development of the sweating mechanism (which in turn required the loss of body hair to be effective) to prevent overheating in the hot savanna environment. Eventually, this sequence of events led to every adaptation of the human lineage, making hunting the defining variable in human evolution (Ardrey 1976; Morris 1967; Brace and Montagu 1965; Montagu 1964).
There are some obvious problems with this argument that make the development of hunting exceedingly unlikely as a primal cause for the development of the modern human thermoregulatory system. First, no matter what the physical evidence for the development of these features, this is a just-so story that was based on speculation first, with evidence forced to fit it second. This idea was developed in a time where the nebulous concept of “the savanna” was not clearly defined. Indeed, the available evidence seems to indicate that the general environment of hominids was marginal forested areas rather than open dry plains (Sikes 1994; WoldeGabriel et al. 1994; Kingston et al. 1994). In addition, the idea of hunting versus scavenging was not much of a consideration at that point in time, eventually became a hotter issue in the 1980s, and has eventually died down with most of the available experimental data supporting scavenging as the mode of hominid animal protein procurement until much later than earlier thought (Blumenschine 1995; Dominguez-Rodrigo 1997).
As another “umbrella hypothesis,” the hunting hypothesis cannot be completely falsified (making its inclusion as an evolutionary hypothesis particularly useless in a variety of ways), but the preconditions that would favour the loss of body hair and the development of a sweating mechanism (high environmental or metabolic thermal stress relative to an earlier state) can be shown to occur prior to when hunting can be reasonably inferred as a likely possibility. Radiation of hominids circa 2 mya indicates a shift into new environments (and the capacity to survive in these environments), and an even earlier increase in body size would indicate increased metabolic stress with the expense of less efficient heat loss through convection with the air. These are events that likely would have involved the need for the development of thermoregulatory changes much earlier than any reasonable evidence of hunting.
Conclusions
Thermoregulation is an important consideration in human evolution. Humans have evolved an extremely efficient mechanism for evaporative cooling that is not seen in other closely related primate species. The development of this sweating mechanism has associated changes in the glandular system of the human skin and the length and thickness of body hair. These changes make sense in terms of a coevolved system of thermoregulation to reduce heat stress on a large-bodied mammal in a hot environment. Any evolutionary hypothesis that attempts to make sense of the thermoregulatory mechanisms discussed above must deal with each of these traits and their distribution in other anthropoids besides humans. A theory that explains the development of trait present in both humans and the African Apes only in the context of unique human evolution and adaptation lacks explanatory power.
Bibliography
Allen, J.A. 1877. “The influence of physical conditions on the genesis of the species.” In Rad. Rev., vol. 1, pp. 108-140.
Amaral, L.Q. 1996. “Loss of body hair, bipedality and thermoregulation. Comments on recent papers in the Journal of Human Evolution.” In Journal of Human Evolution, vol. 30, no. 4, pp. 357-366.
Ardrey, R. 1976. The Hunting Hypothesis. New York: Bantam Books.
Bass, D.E., C.R. Kleeman, and M. Quinn. 1955. “Mechanisms of acclimatization to heat in man.” In Medicine (Baltimore), vol. 34, pp. 323-80.
Bergmann, C. 1847. “Uber die verhaltniesse der warmeokonomie der thiere zu ihrer grosse.” In Göttingen Stud., vol. 1, pp. 393-708.
Blumenschine, R. 1995. “Percussion marks, tooth marks, and experimental determinations of the timing of hominid and carnivore access to long bones at FLK Zinjanthropus, Olduvai Gorge, Tanzania.” In Journal of Human Evolution, vol. 29, pp. 21-51.
Brace, C.L. and A. Montagu. 1965. Human Evolution. New York: Macmillan.
Cabot Briggs, L. 1975. “Environment and human adaptation in the Sahara.” In Physiological Anthropology, ed. by A. Damon, pp. 93-129. New York: Oxford University Press.
Chatterjee, C.C. 1979. “Human physiology.” In Med. Allied Agency, vol. 2, pp. 2-3.
Darwin, C. 1871. The Descent of Man and Selection in Relation to Sex. New York: Modern Library.
Denton, Derek. 1984. The Hunger for Salt: An Anthropological, Physiological, and Medical Analysis. Berlin: Springer-Verlag.
Dominguez-Rodrigo, M. 1997. “Meat-eating by early hominids at the FLK 22 Zinjanthropus site, Olduvai Gorge (Tanzania): An experimental approach using cut-mark data.” In Journal of Human Evolution, vol. 33, no. 6, pp. 669-690.
Ebling, J. 1985. “The mythological evolution of nudity.” In Journal of Human Evolution, vol. 14, pp. 33-41.
Gray, H. 1974. Gray’s Anatomy: Anatomy, Descriptive and Surgical. Philadelphia: Running Press.
Hamilton, W.J., III. 1973. Life’s Color Code. New York: McGraw-Hill Book Company.
Hanna, J., and D. Brown. 1983. “Human heat tolerance: An anthropological perspective.” In Annual Review of Anthropology, vol. 12, pp. 259-284.
Hanson, G., and W. Montagna. 1962. “The skin of primates. XII. The skin of the owl monkey (Aotus trivirgatus).” In American Journal of Physical Anthropology, vol. 20, pp. 421-430.
Hardy, A. 1960. “Was man more aquatic in the past?” In New Scientist, March 17, pp. 642-645.
Holbrook, K.A., and S.I. Minami. 1991. “Hair follicle embryogenesis in the human: Characterization of events in vivo and in vitro.” In Annals of the N.Y. Academies of Science, vol. 642, pp. 167-196.
Holliday, T.W. 1995. “Ecogeographical patterning in body form: Neontological perspectives.” In Body size and proportion in the Late Pleistocene Western Old World and the origins of modern humans, Ph.D. dissertation chapter, pp. 44-66.
Kingston, J.D., B.D. Marino, and A. Hill. 1994. “Isotopic evidence for Neogene hominid paleoenvironments in the Kenya Rift Valley.” In Science, vol. 264, pp. 955-959.
Kligman, A.M. 1988. “The comparative histopathology of male-pattern baldness and senescent balding.” In Clinical Dermatology, vol. 6, no. 4, pp. 108-118.
Kushlan, J.A. 1985. The vestiary hypothesis of human hair reduction.” In Journal of Human Evolution, vol. 14, pp. 29-32.
Langdon, J.H. 1997. “Umbrella hypotheses and parsimony in human evolution: a critique of the Aquatic Ape Hypothesis.” In Journal of Human Evolution, vol. 33, no. 4, pp. 479-494.
Lind, A.R., and D.E. Bass. 1963. “Optimal exposure time for development of acclimatization to heat.” In Fed. Proc., vol. 22, pp. 7.
Mahoney, S.A. 1980. “Cost of locomotion and heat balance during rest and running from 0 to 55°C in a patas monkey.” In Journal of Applied Physiology, vol. 49, pp. 789-800.
Montagna, W. 1976. “General review of the anatomy, growth, and development of hair in man.” In Biology and Disease of the Hair, ed. by. K. Toda, pp. xxi-xxxi. Baltimore: University Park Press.
Montagna, W. 1985. “The Evolution of Human Skin (?)” In Journal of Human Evolution, vol. 14, pp. 3-22.
Montagu, A. 1964. “Natural selection and man’s relative hairlessness.” In Journal of the American Medical Association, vol. 187, pp. 356-357.
Moretti, G., E. Rampini, and A. Rebora. 1976. “The hair cycle re-evaluated.” In International Journal of Dermatology, vol. 15, no. 4, pp. 277-285.
Morgan, E. 1972. The Descent of Woman. New York: Bantam.
Morgan, E. 1982. The Aquatic Ape. New York: Stein and Day.
Morgan, E. 1990. The Scars of Evolution. New York: Oxford University Press.
Morris D. 1967. The Naked Ape. New York: Dell Publishing Co.
Napier, J.R., and P.H. Napier. 1994. The Natural History of Primates. Cambridge: The MIT Press.
Perkins, E.M., and H. Machida. 1967. “The skin of primates. XXXIV. The skin of the golden spider monkey (Ateles geoffroyi).” In American Journal of Physical Anthropology, vol. 26, pp. 35-43.
Riesenfeld, A. 1981. “The role of body mass in thermoregulation.” In American Journal of Physical Anthropology, vol. 55, pp. 95-99.
Robertshaw, D. 1985. “Sweat and heat exchange in man and other mammals.” In Journal of Human Evolution, vol. 14, pp. 63-73.
Ruff, C.B. 1991. “Climate and body shape in hominid evolution.” In Journal of Human Evolution, vol. 21, pp. 81-105.
Sanyal, D.C., and N.K. Maji. 2001. “Thermoregulation through skin under variable atmospheric and physiological conditions.” In Journal of Theoretical Biology, vol. 208, pp. 451-456.
Schultz, A.H. 1931. “The density of hair in primates.” In Human Biology, vol. 3, pp. 303-321.
Schwartz, G.G. and L.A. Rosenblum. 1981. “Allometry of primate hair density and the evolution of human hairlessness.” In American Journal of Physical Anthropology, vol. 55, pp. 9-12.
Senay, L.C., D. Mitchell, and C.H. Wyndham. 1976. “Acclimatization in a hot, humid environment: body fluid adjustments.” In Journal of Applied Physiology, vol. 40, no. 5, pp. 786-96.
Sikes, N.E. 1994. “Early hominid habitat preferences in East Africa: Paleosol carbon isotopic evidence.” In Journal of Human Evolution, vol. 27, pp. 25-45.
Taylor, C.R. 1977. “Exercise and environmental heat loads: different mechanisms for solving different problems?” In (D. Robertshaw, Ed.) Environmental Physiology II. (Int. Rev. Physiol. vol. 15), pp. 119-146.
Van Horn, R. 1970. “Vibrissae structure in the Rhesus monkey.” In Folia Primatologia, vol. 13, pp. 241-285.
Verhaegen, M.J.B. 1985. “The aquatic ape theory: evidence and a possible scenario.” In Med. Hypotheses, vol. 16, pp. 17-32.
Wheeler, P.E. 1984. “The evolution of bipedality and loss of functional body hair in hominids.” In Journal of Human Evolution, vol. 13, pp. 91-98.
Wheeler, P.E. 1985. “The loss of functional body hair in man: the influence of thermal environment, body form and bipedality.” In Journal of Human Evolution, vol. 14, pp. 23-28.
Wheeler, P.E. 1991. “The thermoregulatory advantages of hominid bipedalism in open equatorial environments: the contribution of increased convective heat loss and cutaneous evaporative cooling.” In Journal of Human Evolution, vol. 21, pp. 107-115.
Wheeler, P.E. 1991b. “The influence of bipedalism on the energy and water budgets of early hominids.” In Journal of Human Evolution, vol. 21, pp. 117-136.
Wheeler, P.E. 1992. “The influence of the loss of functional body hair on the water budgets of early hominids.” In Journal of Human Evolution, vol. 23, pp. 379-388.
Wheeler, P.E. 1994. “The thermoregulatory advantages of heat storage and shade seeking behavior to hominids foraging in equatorial savanna environments.” In Journal of Human Evolution, vol. 24, pp. 13-28.
WoldeGabriel, G., T.D. White, G. Suwa, P. Renne, J. de Heinzelin, W.K. Hart, and G. Helken. 1994. “Ecological and temporal placement of early Pliocene hominids at Aramis, Ethiopia.” In Nature, vol. 371, pp. 330-333.